In my studies at Heriot Watt University a great deal of attention was placed on a beer defect called ‘diacetyl’. In fact one of my exam questions called for me to write an essay explaining the diacetyl metabolic pathway. Ever since starting my studies 3 years ago, I have been on the hunt for a beer exhibiting the diacetyl defect. Believe me…I have consumed a lot of beer as part of this hunt. Literature says it is identifiable with its butterscotch candy cloying sweetness. Some brewers have told me its aroma resembles that of a freshly opened can of corn niblets. And last week I finally found an example in a lager from Farmery Brewing in Manitoba which I purchased at the local SLGA agency store in Mossbank, Saskatchewan. When I took the first mouthful of beer, my immediate thought was that Farmery was maybe using a unique yeast strain. As I finished off the can and smelled the aroma of the empty can, my inner beer sense said this was not a yeast strain issue. A couple mouthfuls of the 2nd can….and I had to stop. The butterscotch cloying sweetness on my palate was too much. I knew at that point what I was tasting was diacetyl. On one hand, I felt a sense of elation at finally having discovered a real example of this defect. On the other hand, I started to feel badly for Farmery Brewing as putting out a defective beer can work against one’s brand image.
Let’s take a quick technical look at diacetyl. When yeast is added to the fermenter, the yeast can sense from the osmotic pressure surrounding it that there is fermentable sugar to be eaten. This triggers the yeast to begin the process of producing the microbiological building blocks needed for cellular growth and reproduction. Two building blocks yeast needs are valine and isoleucine. These are amino acids used in the manufacture of cellular proteins. If per chance the yeast cannot find enough of these amino acids in the fermentable barley wort medium, it will synthesize its own valine and isoluecine from carbohydrates (sugars) in the wort medium. This synthesis involves the formation of alpha-keto acids and yeast in its enthusiasm tends to over-produce these acids. The cells will then excrete the alpha acids into the surrounding fermentation wort. The secreted acids are decarboxylated ( a Carbon and two Oxygen atoms are cleaved off making Carbon dioxide) into a chemical substance called diacetyl.
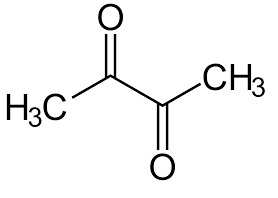
Towards the end of the fermentation cycle, IF the yeast cells are healthy and viable, they will absorb the diacetyl into the yeast cell cytoplasm where it is converted to acetoin and thence to a compound called 2-3-butanediol. This latter compound is virtually impossible for us to taste. The exact reason why yeast will absorb diacetyl remains to be fully understood by science. IF the yeast cells are unhealthy ( perhaps the brewer has serially re-pitched his yeast one too many times…) then the cells will not absorb the diacetyl and the beer will get canned or bottled and sent out into the world with the defect, unless the brewer catches the defect through his quality assurance program.
Now here is where it gets interesting. Diacetyl can also be formed in perfectly good packaged beer if that beer ends up not being properly handled. A chemical reaction called a Maillard reaction can result in the 2-3-butanedione (or acetoin) being oxidized back into diacetyl format. A brewer sending out what he thought was good beer to the SLGA warehouse in Regina, Sask, can have that beer made defective through poor handling practices at the warehouse. We are in the midst of the coronavirus economic shutdown and with a wee bit of imagination I suggest we can all envision a scenario where a lack of employees working at the warehouse led to beer not being properly kept cool.
At the end of the argument, this defect may not be the fault of Farmery Brewing. It could well be the fault of poor handling practices, but such practices may be the result of the coronavirus economic shutdown. There may be nobody to blame.
In any event, if you are looking for a real life example of diacetyl and if your travels take you 40 minutes south of Moose Jaw to Mossbank, Sask, grab a 6-pak of Farmery Lager at the local Food Store. Share the beers with friends so they too can learn what diacetyl tastes like.
For a more academic, technical treatment of diacetyl, the following link will take you to a well written paper from the Journal of the Institute of Brewing.
https://onlinelibrary.wiley.com/doi/epdf/10.1002/jib.84
Cheers! and happy beer drinking….



